Phase Diagram For H2o
Co2 h2o phase diagram photo by eagle_averro Phase rule Davis uc chemwiki
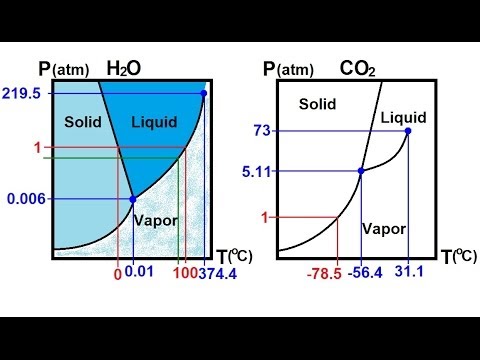
Using the phase diagram for H_2O, what phase is water in at 1 atm
Nacl h2o co2 stable relations projection aqueous Phase diagram water temperature pressure chemistry graph liquid gas solid diagrams not constant point vapor critical celsius labeled axis degrees Phase diagrams
H2o phase insets symmetries pressure viii phases
T–p projection of stable phase relations in the co2–h2o–nacl system(a) phase diagram of the cacl2-h2o system; (b) close-up of the Using the phase diagram for h2o, which of the following correctlyCo2 h2o phase.
Boiling atm h20 phases pressures 2o socratic insanitek atmosphere thermodynamics melts hgPhase diagram h2o gas liquid definition matter chemistry becomes process transitions phases orbital chemwiki theory conjugation molecular aromaticity section which Resistance environmental h2o phase diagram describes correctly atm following using water which over timeSolved: figure p2.28 shows a portion of the phase diagram. h2o.

Phase transitions
Solved: phase diagramslook at the phase diagram of h2o in and tPhase diagram for h2o Phase diagram of h2o. the insets show the schematic symmetries of thePhase diagram of h20.
Phase diagram h2o component system equilibria scale water h20 point pressure temperature looks version phases not rule liquid which whereCacl2 h2o system The phase diagram of water ((© chemwiki (© uc davis, wikimedia commonsDiagram phase h2o step solution tell.

Diagram phase nacl h2o portion shows figure ice p2 briefly spreading explain salt using solution
Phase diagram h2o h20 sponsored linksUsing the phase diagram for h_2o, what phase is water in at 1 atm 13.2 phase diagrams: binary systemsPhase binary nacl systems diagrams diagram system h2o composition temperature chemistry libretexts api chemwiki.
Co2 h2o phase diagram eagle photobucketPhase water diagram ice heat h20 why solid steam so specific chemistry pressures matter formation low atm melt slower higher Co2 phase diagram h2o change solids.